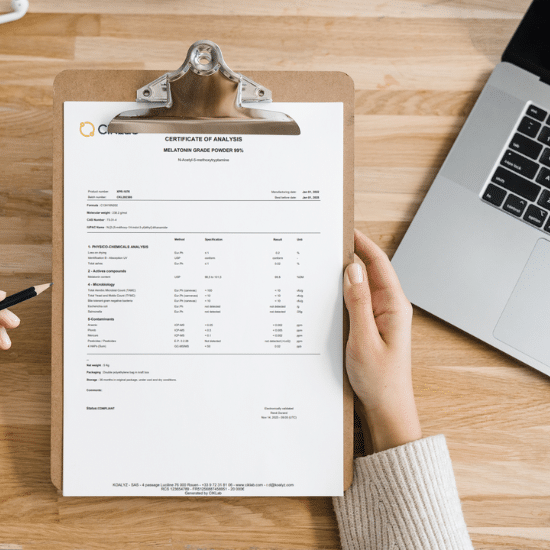
What is a Certificate of Analysis (CoA) in the nutraceutical industry?
"Nothing looks more alike than two white powders. And nothing is more deceptive…"
A Certificate of Analysis is far more than a routine document. It provides documented evidence that a product meets defined quality, performance, and safety criteria. In the ingredients, dietary supplements, and nutraceutical sectors, CoAs are systematically requested in B2B transactions between suppliers and buyers.
A CoA typically complements the technical data sheet and serves as a key reference before contractual agreements are finalized. It enables both parties to verify critical parameters that define the product being purchased and the batch actually delivered. In practice, each production batch should be accompanied by its own CoA.
Certificates of Analysis are not mere administrative paperwork; they are a primary proof point that products comply with technical, regulatory, and safety specifications. They are a cornerstone of trust for manufacturers, distributors, and ultimately, consumers.

Why are Certificates of Analysis essential in the nutraceutical industry?
Complexity requires precision
The nutraceutical industry is marked by an exceptional diversity of raw materials, many of which exhibit significant natural variability; botanical extracts, technical blends, probiotics, vitamins, minerals, and amino acids. These materials are not static; their chemical, physical, and microbiological profiles can vary depending on origin, processing, and intended use. Some botanicals also contain naturally occurring toxic compounds, such as certain alkaloids, that must be carefully monitored.
"Few industries require this level of analytical rigor. From an analytical standpoint, nutraceuticals sit somewhere between food, chemicals, and pharmaceuticals, which explains the need for advanced testing approaches:"
- Verify substance identity, for example authentication of plant extracts via PCR, phytochemical profiling, or isotopic analysis.
- Control geographical origin where relevant through isotopic traceability.
- Detect adulteration, including dilution, substitution, or mislabeling of raw materials.
- Monitor contaminants such as natural toxins, pesticides, heavy metals, MOSH/MOAH, and solvent residues.
- Precisely quantify active compounds, including curcuminoids, polyphenols, caffeine, or probiotic counts.
💡 → In short, analytical rigor is not optional; it is the foundation of product integrity.

1. Generate trust through reliable analytical results
The primary objective of a Certificate of Analysis is to provide accurate, reliable, and verifiable data. This relies on:
- The use of validated analytical methods such as ISO, AOAC, Ph. Eur., or USP.
- Transparency regarding the techniques employed, for example HPLC for actives or ICP-MS for heavy metals.
- Alignment with internationally recognized standards, which facilitates trade, ensures comparability, and strengthens credibility.
💡 → A well-constructed CoA reassures customers and demonstrates that product quality is actively controlled, not assumed.
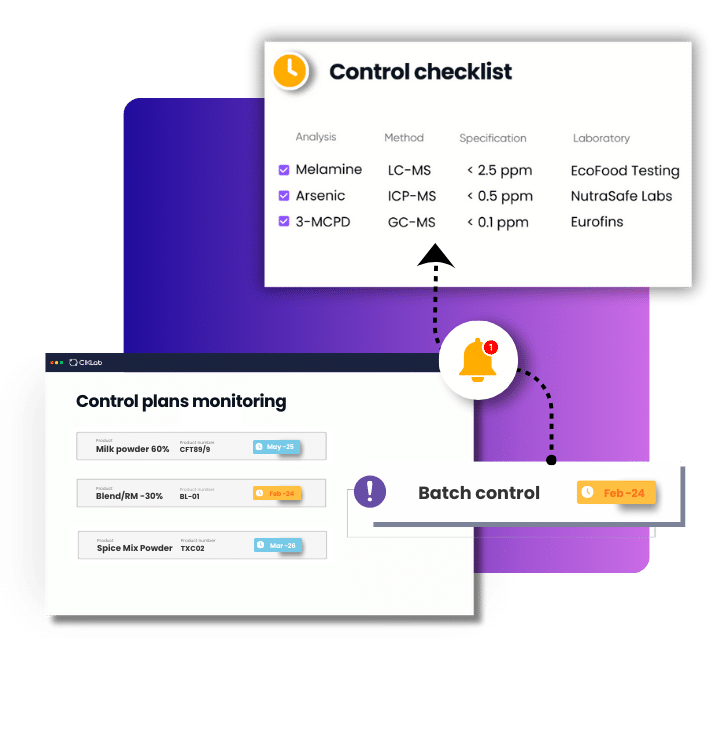
CIKLab is software specialized in nutraceutical quality control and Certificate of Analysis editing
2. Clarify intended use and formulation
A Certificate of Analysis provides data that directly supports product formulation and regulatory compliance by:
- Enabling formulators to design products based on verified raw material characteristics such as protein content, active compounds, or lipid profile.
- Ensuring alignment between label claims and actual analytical results.
3. Demonstrate quality, technical performance, and food safety
A Certificate of Analysis documents that products meet established specifications defined in the control plan, including:
- Quality attributes such as minimum active content or guaranteed levels.
- Functional characteristics such as solubility, stability, or dispersibility.
- Safety parameters including microbial toxins, chemical contaminants, pesticides, heavy metals, and allergens.
💡 → Without robust analytical documentation, demonstrating compliance becomes significantly more difficult.
4. Support regulatory compliance
The CoA serves as evidence of conformity with applicable regulatory frameworks, including FDA requirements, EU regulations, and national guidelines. Certain markets explicitly require CoAs containing specific data, for example microbiology, heavy metals, allergens, or GMO status.
5. Ensure traceability and manage non-conformities
Each Certificate of Analysis is linked to a unique production batch number. In the event of a complaint, deviation, or recall, this linkage enables precise traceability throughout the supply chain and helps identify affected batches efficiently.
💡 → Traceability remains a core pillar of food safety; the CoA is one of its most visible components.
6. Enable continuous improvement and innovation
Systematic analysis of CoA data allows companies to:
- Identify trends and potential quality drifts.
- Optimize manufacturing processes.
- Support R&D efforts, such as improving extraction methods or selecting superior plant varieties.
💡 → In practice, CoAs are not just compliance documents; they are a valuable source of structured quality intelligence.
What should a Certificate of Analysis include in the nutraceutical industry?
In nutraceuticals, some Certificates of Analysis function as hybrids between analytical reports and certificates of conformity, as they may include statements that go beyond pure laboratory testing. They often mirror elements of the product specification sheet.
"The real challenge in producing high-quality CoAs lies not in formatting but in having a robust quality control structure that ensures full traceability from the control plan to batch release."
1. CoA Header
The header establishes the document's authenticity and credibility. It should include:
- Company identity and logo, whether supplier or manufacturer.
- Clear title; "Certificate of Analysis".
- Unique CoA number for traceability and archiving.
- Issue date and document version if applicable.
2. General product and batch information
This section defines exactly what has been analyzed, typically including:
| Information Category | Key Elements |
|---|---|
| Identification | Unique product reference and batch number. |
| Key dates | Harvest, manufacturing, packaging, expiry, and analysis or retest dates. |
| Technical details | Latin name, CAS or EINECS numbers, geographical origin, and plant part used. |
| Process information | Extraction or drying method, solvents used, carriers, ratios, or additives. |
3. Analytical results — the core of the CoA
This is the most critical part of the document; clear presentation here equals transparency and professionalism.
- Using explicit analysis names and avoiding ambiguous abbreviations.
- Specifying exact analytical methods such as ISO, AOAC, Ph. Eur., or USP; if internal methods are used, this must be clearly stated.
- Defining acceptance criteria unambiguously to avoid interpretation issues.
- Always including units of measurement such as %, mg/kg, or CFU/g.
- Clearly indicating whether results are compliant or non-compliant.
Typical analysis categories include:
| Analysis Type | Examples |
|---|---|
| General characteristics | Appearance, color, odor, pH, density |
| Physico-chemical tests | Moisture, ash, loss on drying, active content |
| Microbiology | Aerobic plate count, yeast and mold, pathogens |
| Natural toxins | Pyrrolizidine alkaloids, aristolochic acids |
| Harvest or storage contaminants | Mycotoxins |
| Environmental contaminants | Heavy metals, pesticides, dioxins, PCBs, PAHs |
| Process contaminants | Solvent residues, MOSH/MOAH, acrylamide |
4. Additional information
Depending on context, a CoA may also include:
- Recommended storage conditions.
- Use precautions or restrictions.
- Additional certifications such as Organic, Halal, Kosher, Non-GMO, or Vegan.
- Allergen declarations.
5. Validation and signature
This section identifies the person responsible for approving the batch and results. It should include name, role, date, and time.
💡 → Signing a Certificate of Analysis engages the company's responsibility. A signature without proper verification can have serious legal and financial consequences.
💡 → A common statement on modern CoAs is: "This document has been generated and validated electronically."
💡 → This helps secure the process, reduce human error, and ensure data integrity, which is now standard practice in pharmaceutical, food, and chemical industries.
How to critically evaluate a supplier's Certificate of Analysis?
Receiving a CoA is good; evaluating it properly is better. Here is a structured four-step approach.
1. Verify content and consistency
Check that the CoA includes all essential sections such as header, batch identification, results, and validation, and confirm that:
- The batch number matches the delivered product.
- Analytical methods are clearly stated and recognized.
- Results align with contractually agreed specifications.
- Consistency of dates, including manufacturing, analysis, and expiry.
- Clear and correct units of measurement.
- Results that meet expected criteria.
2. Qualify both the document and the supplier
A CoA alone is not always sufficient. Key questions include:
- Is it signed by an authorized person?
- Is the laboratory accredited, for example ISO 17025 or GMP?
- Are the methods validated and appropriate?
- Is there clear traceability between samples and batches?
3. Involve your quality team
Supplier CoAs should be systematically reviewed before production. A formal validation process should include:
- A documentary compliance checklist.
- Alert thresholds triggering confirmatory testing.
- Periodic supplier audits when justified by risk or volume.
4. Assess the supplier's quality system
A CoA reflects the system that produced it. To evaluate reliability:
- Request regular quality audits.
- Review accreditation reports from subcontracted laboratories.
- Analyze historical CoA data for recurring deviations.
- Check how frequently analytical methods are updated.
Ultimately, apply to your suppliers the same level of rigor you expect from your own quality system; you are accountable for what you purchase.
Why use CIKLab for managing Certificates of Analysis?
Throughout this article, one point is clear; in nutraceuticals, the Certificate of Analysis is not just another document; it is one of the most visible components of how you are perceived by your clients and the market. It reflects your analytical choices, your risk management, your traceability, and your credibility with customers and regulators.
This is exactly where CIKLab comes in.
In an industry where precision, traceability, and flexibility are critical, a dedicated quality control management system that naturally generates Certificates of Analysis is far more effective than simple document templates.

Using standalone tools like Excel or Word to "generate" CoAs, without linking them to a structured control plan and batch management system, creates manual re-entry, scattered files, and significant risks of inconsistency.
CIKLab ensures that what you plan, what you test, and what you deliver on the CoA are perfectly aligned.
| Challenge | CIKLab Solution |
|---|---|
| Multiple analytical methods (HPLC, LC-MS, microbiology, contaminants) from internal, external, and supplier sources | A centralized analysis catalog linked to control plans |
| Raw material heterogeneity and result variability | Configurable control strategies by product, material, risk level, and supplier |
| Risk of re-entry errors | Single data entry with automatic reuse in CoAs |
| Inconsistencies between control plan and delivered CoA | Native connection between control plans, batches, and certificates |
| Difficulty managing testing periodicities (batch-by-batch, annual, quarterly) | Automated management of testing periodicities |
| Insecure CoA signatures (modifiable Word/PDF files) | Secure electronic signatures, role-based access, and full audit trail |
| Time wasted formatting different CoA models per customer | Customizable CoA templates per customer and regulation |
| Difficulty tracing back during audits | Complete traceability from control plan to results, quality decision, and archived CoA |
| Complex collaboration with subcontracted laboratories | Structured integration of external laboratory results |
| Dispersed and insecure CoA archiving | Secure, searchable CoA repository |
| Difficulty analyzing quality trends over time | Data analytics to monitor quality trends over time |