Quality Control Software
for Food Ingredients
Industry 🍃
Finally master hundreds of control plans, no matter your raw materials, product specifications, or suppliers.







Ingredient Industry:
Mastering the Complexity of Quality Control
In the food ingredients industry (nutraceuticals, additives, flavors, plant extracts, etc.), regulatory requirements, the diversity of raw materials and their origins, and the sheer number of analyses to be performed make quality control particularly complex. This means managing a multitude of control and monitoring plans!
Few industries demand such a wide range of technical and analytical expertise to characterize raw materials: purity, authenticity, botanical identification, substances to monitor, natural endogenous contaminants… each step requires true expertise.
CIKLab is designed to structure, automate, and track your entire quality control process, from the receipt of raw materials to batch release and the automatic generation of Certificates of Analysis.

How our Quality Control Software Helps You
In the food, nutraceutical, and functional ingredient industry, the diversity of raw materials, suppliers, and analytical requirements makes quality control difficult to structure and secure.
CIKLab provides a concrete answer to your main challenges
Too Many Control Plans!
Complex and variable technical specifications
Each ingredient comes with its own physico-chemical, microbiological, and contaminant criteria that must be verified upon every delivery. Managing these requirements manually increases the risk of omissions and non-compliance.
- Control plans configured by ingredient and supplier
- Automatic application of analyses and controls for each received batch based on specifications and testing frequencies
- Clear visibility of expected, completed, and missing analyses through history tracking and automated reminders
Multiple Suppliers
Multiple suppliers, sources, and origins
Raw materials sourced worldwide mean multiple specifications to manage, different regulations to comply with, and customer requirements to follow simultaneously.
- Centralized specifications by supplier and raw material
- Complete quality history by origin and batch
- Easy comparison of supplier performance
Highly Specialized Analyses
Multiple outsourced laboratories
Microbiology, pesticides, heavy metals, analytical profiles… most analyses are outsourced to specialized laboratories, making tracking fragmented and difficult to manage.
- Analysis and laboratory catalog
- Pre-selection of laboratories by analysis type or matrix
- Real-time tracking of internal and external analyses
Certificates of Analysis for Every Batch
Multiple data sources & complex consolidation
In the ingredient industry, CoAs often combine data from multiple sources: suppliers, periodic testing, external and internal laboratories, and additional information. Manual consolidation is time-consuming, complex, and error-prone.
- CoAs configured for each product type
- Automatic centralization of all analytical data
- Reliable one-click CoA generation: dated, signed, and fully traceable
Simplify Your Quality Control and Save Time!
Discover how CIKLab can transform ingredient quality management
14-Day Free TrialCertificates of Analysis
In the Food Ingredients industry, every batch sold requires a complete and reliable Certificate of Analysis (CoA). These documents consolidate analytical data from multiple sources: suppliers, external laboratories, periodic internal testing, and additional information.
Manual compilation is time-consuming, complex, and prone to errors.
Time and Efficiency Savings
Generate your Certificates of Analysis with a single click thanks to full process automation.
- Instant automatic CoA generation
- Complete elimination of manual entry errors
- No more tedious data reprocessing
- Up to 70% reduction in CoA production time
Centralization and Traceability
All your analytical data on a single platform, accessible anytime.
- Centralized management of internal and external analyses
- Complete history of all generated certificates
- Instant access to CoAs from anywhere
- Full audit trail of all changes
Security and Compliance
Meet regulatory requirements with electronic signatures and rights management.
- Automatic electronic signature and timestamping
- Granular user permissions management
- Tamper-proof, dated documents
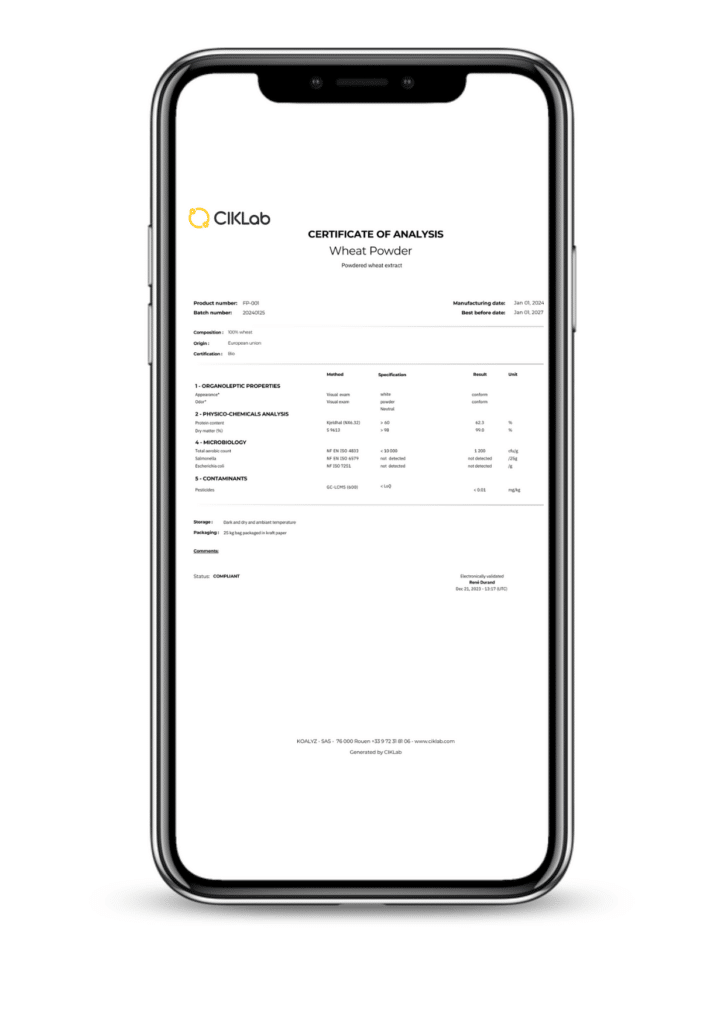
The Result: Reliable CoAs, Quickly Available
Try CIKLab today for free !
Discover how to simplify quality control for food ingredients